
Unmet Clinical Need
• Large diabetic population with small vessels & diffused disease
• A dedicated small diameter, long DES.
• Once and done. Allows cost reduction.

Stent architecture
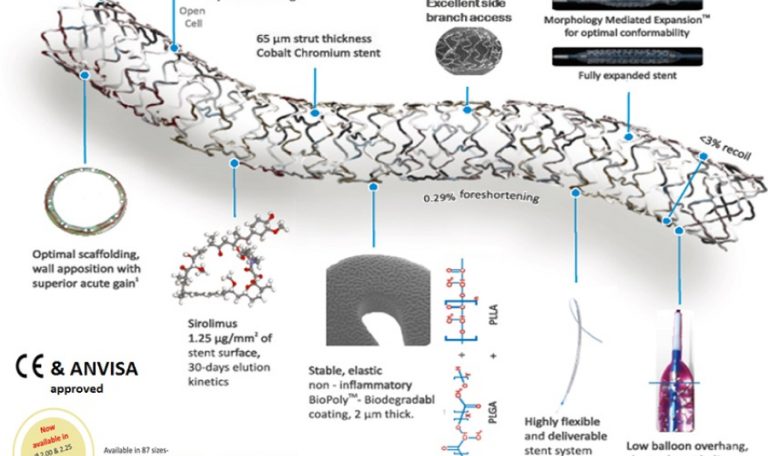
• Cobalt chromium (L605) platform with 65µm strut thickness.
• Hybrid cell design comprising of an intelligent mix of open and close cells
resulting in excellent radial strength with a high flexibility.
• Unique strut width variability that ensure a <3% recoil and 0.29%
foreshortening.
• Special electro-polishing technique eliminates surface nickel oxides.

Creative, colour coded spine labeling for easy retrieval from shelves
Low injury Design – Morphology Mediated ExpansionTM
• Conventional edge-flaring stent designs allow the stent to
dog-bone during deployment.
• This dog-boning coupled with balloon overhang may cause
edge injury.
• BioMime has struts with design variability which results in
morphology mediated expansionTM, having a propensity to
minimize stent edge injury.
• High fatigue resistance.
• High radial force.
• Excellent side branch access.
• Best in class crossing profile 0.033”.
• Enhanced delivery system for better Trackability, Pushability
and Torqueability.

Novel Drug Release Platform
• Biomime Aura DES uses a validated formulation of low dose sirolimus
(1.25 µg/mm²) times to elute in ~ 30 days from a biodegradable polymer
base which degrades simultaneously.
MeriStemTM
• Novel Shaft material for increased strength and kink resistance.
FeatherGlideTM
• Seamless catheter construction technology allows for exceptionally low
tracking force of 0.35N.
• Construction which generates high push transfer capability and reducing
the push forces to less than 0.08N.


